Dr. Laura Molina, PittMed MSTP and first year Pathology Resident at UPMC publishes her first Senior author study along with PLRC members Dr. Paul Monga, MD, Silvia Liu, PhD, Kari Nejak-Bowen, PhD, MBA, andXiaochao Ma, PhD. The article is published in Hepatology Communications and is entitled,
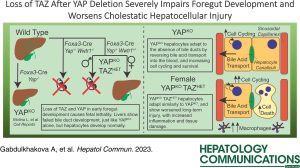
“Loss of TAZ after YAP deletion severely impairs foregut development and worsens cholestatic hepatocellular injury”.
Gabdulkhakova A, Krutsenko Y, Zhu J, Liu S, Poddar M, Singh S, Ma X, Nejak-Bowen K, Monga SPS, Molina LM. Loss of TAZ after YAP deletion severely impairs foregut development and worsens cholestatic hepatocellular injury. Hepatol Commun. 2023 Aug 9;7(9):e0220. doi: 10.1097/HC9.0000000000000220. PMID: 37556373.
Abstract
Background: We previously showed that loss of yes-associated protein 1 (YAP) in early liver development (YAPKO) leads to an Alagille syndrome-like phenotype, with failure of intrahepatic bile duct development, severe cholestasis, and chronic hepatocyte adaptations to reduce liver injury. TAZ, a paralog of YAP, was significantly upregulated in YAPKO hepatocytes and interacted with TEA domain family member (TEAD) transcription factors, suggesting possible compensatory activity.
Methods: We deleted both Yap1 and Wwtr1 (which encodes TAZ) during early liver development using the Foxa3 promoter to drive Cre expression, similar to YAPKO mice, resulting in YAP/TAZ double knockout (DKO) and YAPKO with TAZ heterozygosity (YAPKO TAZHET). We evaluated these mice using immunohistochemistry, serum biochemistry, bile acid profiling, and RNA sequencing.
Results: DKO mice were embryonic lethal, but their livers were similar to YAPKO, suggesting an extrahepatic cause of death. Male YAPKO TAZHET mice were also embryonic lethal, with insufficient samples to determine the cause. However, YAPKO TAZHET females survived and were phenotypically similar to YAPKO mice, with increased bile acid hydrophilicity and similar global gene expression adaptations but worsened the hepatocellular injury. TAZ heterozygosity in YAPKO impacted the expression of canonical YAP targets Ctgf and Cyr61, and we found changes in pathways regulating cell division and inflammatory signaling correlating with an increase in hepatocyte cell death, cell cycling, and macrophage recruitment.
Conclusions: YAP loss (with or without TAZ loss) aborts biliary development. YAP and TAZ play a codependent critical role in foregut endoderm development outside the liver, but they are not essential for hepatocyte development. TAZ heterozygosity in YAPKO livers increased cell cycling and inflammatory signaling in the setting of chronic injury, highlighting genes that are especially sensitive to TAZ regulation.