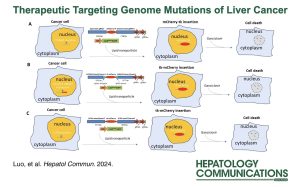
Dr. Silvia Liu and Dr. Jianhua Luo the Directors of the Genomics and Systems Biology Core of the PLRC published an article in Hepatology Communications entitled, “Therapeutic targeting at genome mutations of liver cancer by the insertion of HSV1 thymidine kinase through Cas9-mediated editing.“
Kader M, Sun W, Ren BG, Yu YP, Tao J, Foley LM, Liu S, Monga SP, Luo JH. Therapeutic targeting at genome mutations of liver cancer by the insertion of HSV1 thymidine kinase through Cas9-mediated editing. Hepatol Commun. 2024 Mar 18;8(4):e0412. doi: 10.1097/HC9.0000000000000412. PMID: 38497929; PMCID: PMC10948134.
 Abstract
Abstract
Background:
Liver cancer is one of the most lethal malignancies for humans. The treatment options for advanced-stage liver cancer remain limited. A new treatment is urgently needed to reduce the mortality of the disease.
Methods:
In this report, we developed a technology for mutation site insertion of a suicide gene (herpes simplex virus type 1- thymidine kinase) based on type II CRISPR RNA-guided endonuclease Cas9-mediated genome editing to treat liver cancers.
Results:
We applied the strategy to 3 different mutations: S45P mutation of catenin beta 1, chromosome breakpoint of solute carrier family 45 member 2-alpha-methylacyl-CoA racemase gene fusion, and V235G mutation of SAFB-like transcription modulator. The results showed that the herpes simplex virus type 1-thymidine kinase insertion rate at the S45P mutation site of catenin beta 1 reached 77.8%, while the insertion rates at the breakpoint of solute carrier family 45 member 2 – alpha-methylacyl-CoA racemase gene fusion were 95.1%–98.7%, and the insertion at V235G of SAFB-like transcription modulator was 51.4%. When these targeting reagents were applied to treat mouse spontaneous liver cancer induced by catenin beta 1S45P or solute carrier family 45 member 2-alpha-methylacyl-CoA racemase, the mice experienced reduced tumor burden and increased survival rate. Similar results were also obtained for the xenografted liver cancer model: Significant reduction of tumor volume, reduction of metastasis rate, and improved survival were found in mice treated with the targeting reagent, in comparison with the control-treated groups.
Conclusions:
Our studies suggested that mutation targeting may hold promise as a versatile and effective approach to treating liver cancers.