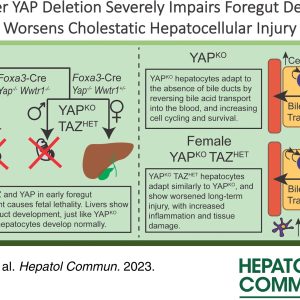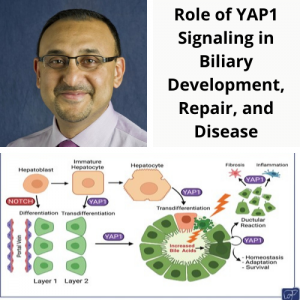
Dr. Joe Locker, MD, PhD along with Ira Fox, MD and Michael Oertel, PhD published and article in Cellular & Molecular Gastroenterology and Hepatology entitled “Mechanism and Effect of HNF4α Decrease in a Rat Model of Cirrhosis and Liver Failure“.
Melis M, Marino R, Tian J, Johnson C, Sethi R, Oertel M, Fox IJ, Locker J. Mechanism and Effect of HNF4α Decrease in a Rat Model of Cirrhosis and Liver Failure. Cell Mol Gastroenterol Hepatol. 2024;17(3):453-479. doi: 10.1016/j.jcmgh.2023.11.009. Epub 2023 Nov 21. PMID: 37993018; PMCID: PMC10837635.
Abstract
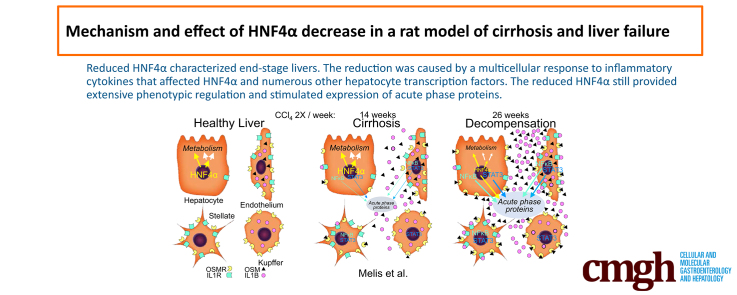
Background & aims: HNF4α, a master regulator of liver development and the mature hepatocyte phenotype, is down-regulated in chronic and inflammatory liver disease. We used contemporary transcriptomics and epigenomics to study the cause and effects of this down-regulation and characterized a multicellular etiology.
Methods: Progressive changes in the rat carbon tetrachloride model were studied by deep RNA sequencing and genome-wide chromatin immunoprecipitation sequencing analysis of transcription factor (TF) binding and chromatin modification. Studies compared decompensated cirrhosis with liver failure after 26 weeks of treatment with earlier compensated cirrhosis and with additional rat models of chronic fibrosis. Finally, to resolve cell-specific responses and intercellular signaling, we compared transcriptomes of liver, nonparenchymal, and inflammatory cells.
Results: HNF4α was significantly lower in 26-week cirrhosis, part of a general reduction of TFs that regulate metabolism. Nevertheless, increased binding of HNF4α contributed to strong activation of major phenotypic genes, whereas reduced binding to other genes had a moderate phenotypic effect. Decreased Hnf4a expression was the combined effect of STAT3 and nuclear factor kappa B (NFκB) activation, which similarly reduced expression of other metabolic TFs. STAT/NFκB also induced de novo expression of Osmr by hepatocytes to complement induced expression of Osm by nonparenchymal cells.
Conclusions: Liver decompensation by inflammatory STAT3 and NFκB signaling was not a direct consequence of progressive cirrhosis. Despite significant reduction of Hnf4a expression, residual levels of this abundant TF still stimulated strong new gene expression. Reduction of HNF4α was part of a broad hepatocyte transcriptional response to inflammation.