Andrew Feranchak, MD, Chief of Pediatric Gastroenterology and Associate director of PLRC, along with PLRC leaders Aatur Singhi, MD, Silvia Liu, PhD and Paul Monga, MD, published an article in American Journal of Physiology: Gastrointestinal Liver Physiology entitled,
“TMEM16A partners with mTOR to influence pathways of cell survival, proliferation and migration in cholangiocarcinoma“.
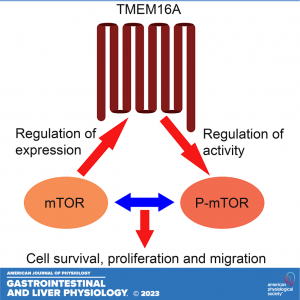
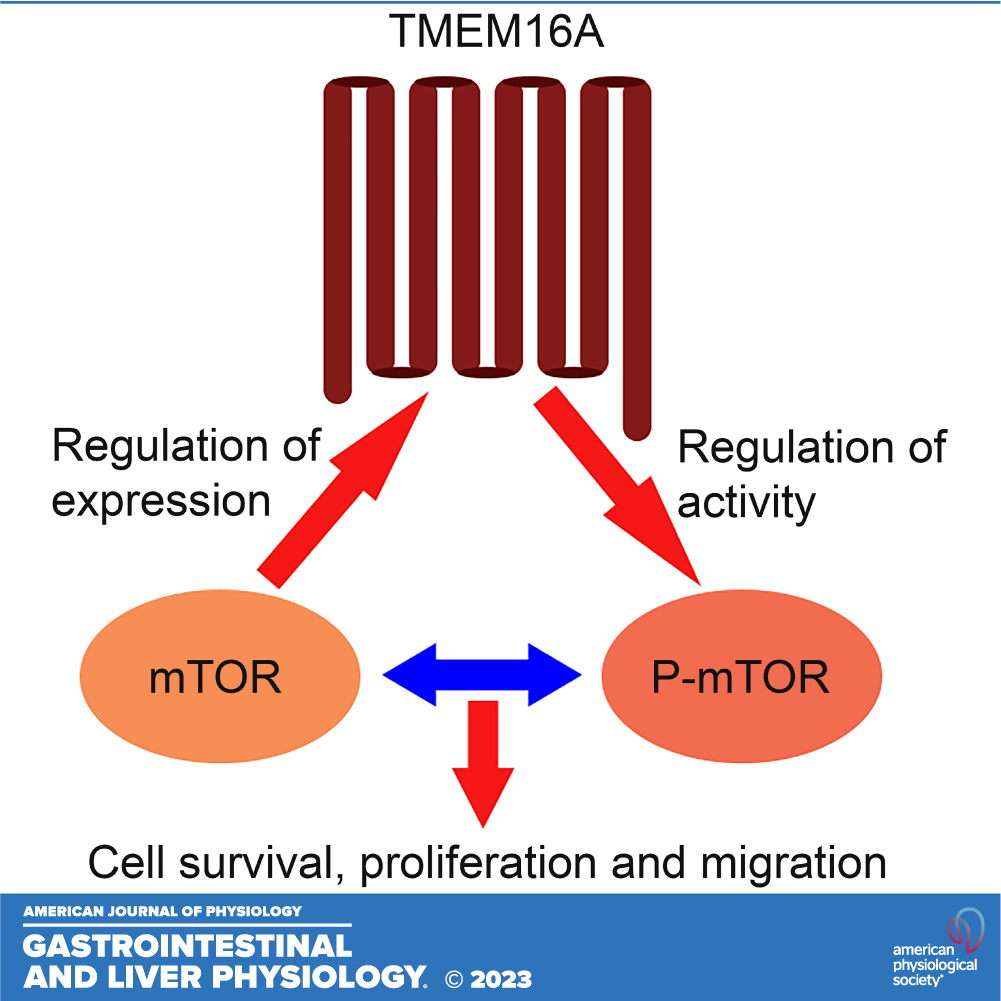
Expression of transmembrane protein 16 A (TMEM16A), a calcium activated chloride channel, is elevated in some human cancers and impacts tumor cell proliferation, metastasis, and patient outcome. Evidence presented here uncovers a molecular synergy between TMEM16A and mechanistic/mammalian target of rapamycin (mTOR), a serine-threonine kinase that is known to promote cell survival and proliferation in cholangiocarcinoma (CCA), a lethal cancer of the secretory cells of bile ducts. Analysis of gene and protein expression in human CCA tissue and CCA cell line detected elevated TMEM16A expression and Cl− channel activity. The Cl− channel activity of TMEM16A impacted the actin cytoskeleton and the ability of cells to survive, proliferate, and migrate as revealed by pharmacological inhibition studies. The basal activity of mTOR, too, was elevated in the CCA cell line compared with the normal cholangiocytes. Molecular inhibition studies provided further evidence that TMEM16A and mTOR were each able to influence the regulation of the other’s activity or expression respectively. Consistent with this reciprocal regulation, combined TMEM16A and mTOR inhibition produced a greater loss of CCA cell survival and migration than their individual inhibition alone. Together these data reveal that the aberrant TMEM16A expression and cooperation with mTOR contribute to a certain advantage in CCA.
NEW & NOTEWORTHY This study points to the dysregulation of transmembrane protein 16 A (TMEM16A) expression and activity in cholangiocarcinoma (CCA), the inhibition of which has functional consequences. Dysregulated TMEM16A exerts an influence on the regulation of mechanistic/mammalian target of rapamycin (mTOR) activity. Moreover, the reciprocal regulation of TMEM16A by mTOR demonstrates a novel connection between these two protein families. These findings support a model in which TMEM16A intersects the mTOR pathway to regulate cell cytoskeleton, survival, proliferation, and migration in CCA.